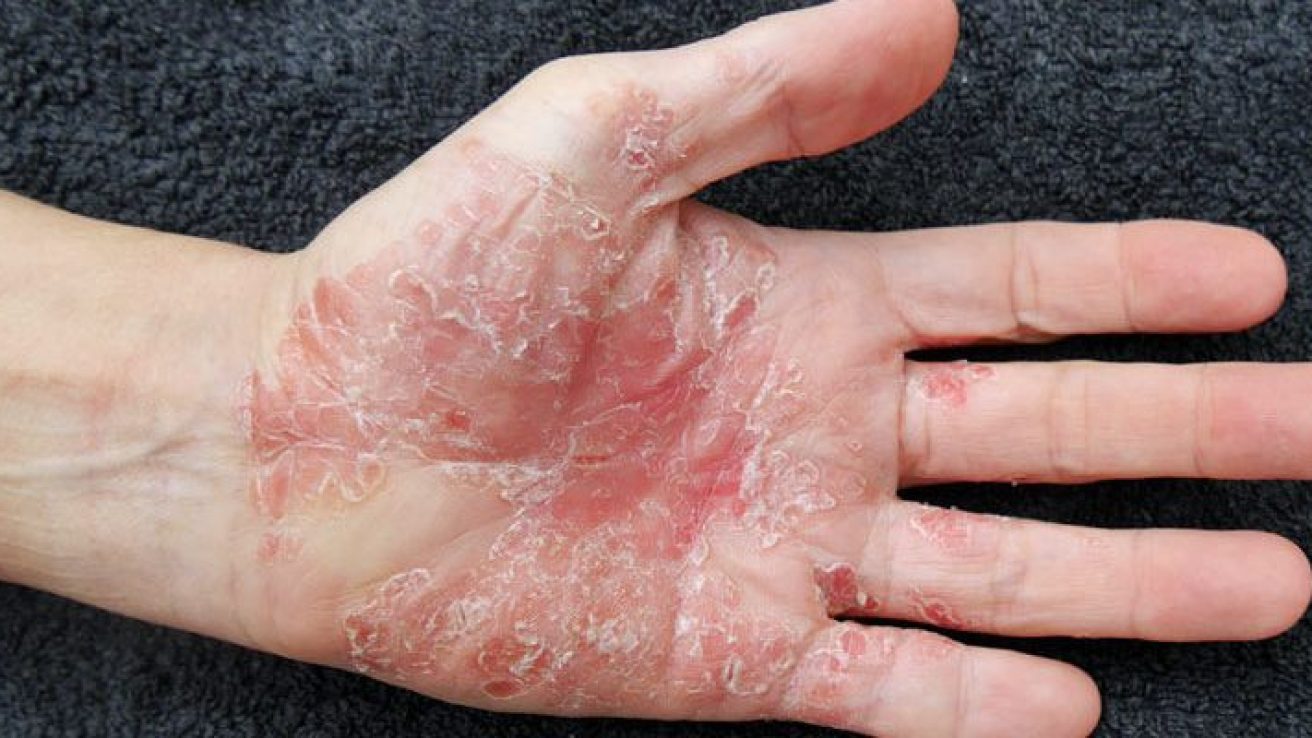
FRIDAY, Feb. 5, 2021 (HealthDay News) — For moderate-to-severe plaque psoriasis, bimekizumab is more efficacious than ustekinumab and placebo, according to a study published in the Feb. 6 issue of The Lancet.
Kristian Reich, M.D., from the University Medical Center Hamburg-Eppendorf in Germany, and colleagues compared the efficacy and safety of bimekizumab with placebo and ustekinumab in patients with moderate-to-severe plaque psoriasis. A total of 567 patients were enrolled and randomly assigned to bimekizumab 320 mg every four weeks (321 patients), ustekinumab 45 or 90 mg at weeks 0 and 4 and then every 12 weeks (163 patients), or placebo every four weeks (83 patients). At week 16, patients receiving placebo switched to bimekizumab.
The researchers found that 85 percent of patients in the bimekizumab group, 50 percent in the ustekinumab group, and 5 percent in the placebo group had 90 percent improvement in the Psoriasis Area and Severity Index at week 16 (risk differences, 35 and 80, respectively). An Investigator’s Global Assessment response occurred in 84, 53, and 5 percent of patients in the bimekizumab, ustekinumab, and placebo groups, respectively, at week 16 (risk differences, 30 and 79, respectively). Serious treatment-emergent adverse events were reported in 6 and 8 percent of patients in the bimekizumab and ustekinumab groups, respectively, over 52 weeks.
“Bimekizumab was efficacious at treating patients with moderate-to-severe plaque psoriasis,” the authors write. “Faster onset of clinically meaningful responses was observed with bimekizumab compared with both ustekinumab and placebo, with responses observed at week four (after one dose).”
Several authors disclosed financial ties to pharmaceutical companies, including UCB Pharma, which manufactures bimekizumab and funded the study.
Abstract/Full Text (subscription or payment may be required)