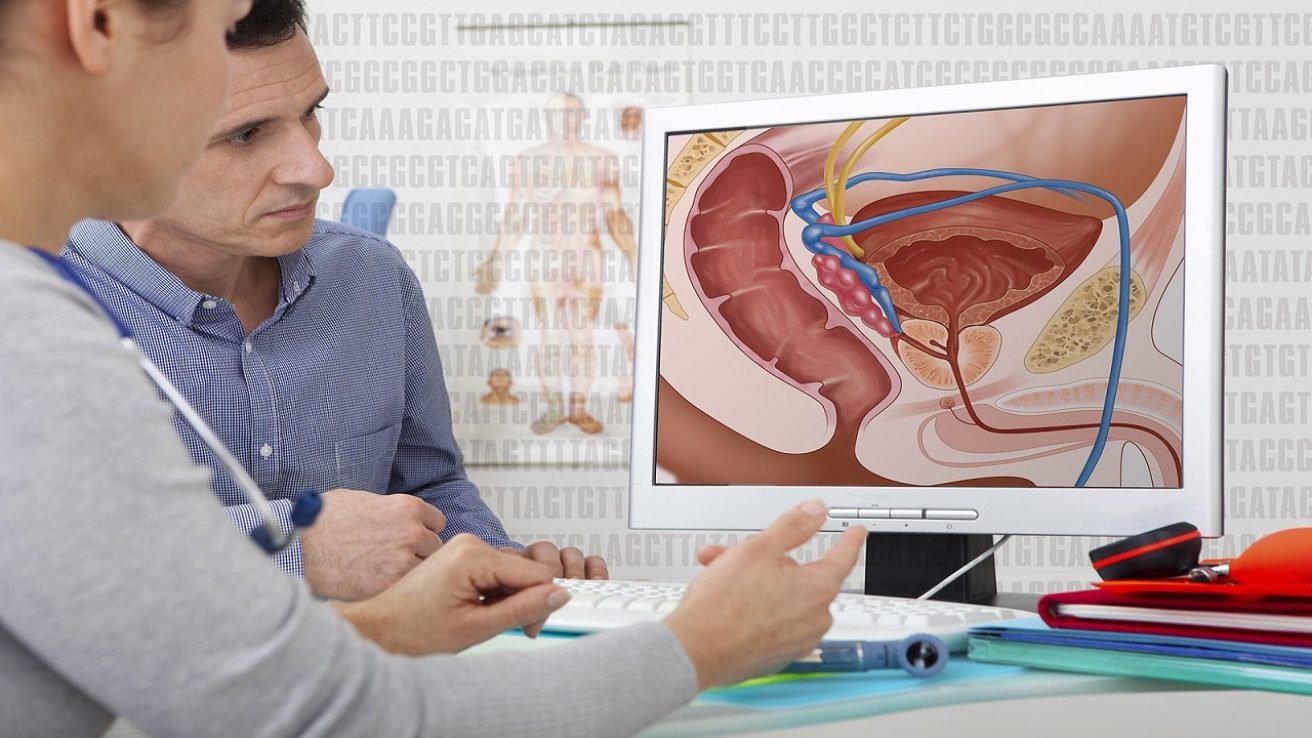
In the united states, there will be approximately 192,000 cases of prostate cancer in 2020 and 33,000 deaths. 1 Clinical trials are often sought in advanced cases of cancer when standard therapy is not working or not an option. Clinical trials have the benefit of providing patients with access to therapies not routinely available.
Immunotherapy
In recent years, immunotherapy has gained popularity in successfully managing various cancers. Immunotherapy works by creating the body’s immune system to function toward preventing, controlling, and eliminating the cancer.
Currently, available FDA approved immunotherapy treatments for prostate cancer include Sipuleucel-T and Pembrolizumab (commonly known as Keytruda). Sipuleucel-T is a vaccine that targets the prostatic acid phosphatase protein which is highly expressed on prostate cancers. Pembrolizumab is a checkpoint inhibitor that targets PD-1/PD-L1 pathway.1
Several current clinical trials are evaluating immunotherapy in combination with other medications improved management of patients with advanced prostate cancer. Current trials include:2
- Pembrolizumab + Olaparib versus abiraterone Acetate or Enzalutamide in Metastatic Castration-resistant Prostate Cancer.
Phase 3 trial, National Clinical Trial Number (NTC): NCT03834519
- Pembrolizumab Plus Docetaxel Versus Placebo Plus Docetaxel in chemotherapy -naïve metastatic castration-resistant prostate cancer.
Phase 3 trial, NCT number: NCT03834506
- Pembrolizumab plus enzalutamide plus androgen deprivation therapy versus placebo plus enzalutamide plus ADT in men with metastatic hormone-sensitive prostate cancer.
Phase 3 trial, NCT number: NCT04191096
- Pembrolizumab combination therapies in metastatic cancer
Phase 1 trial, NCT number: NCT02861573
- Pembrolizumab in participants with metastatic castration-resistant prostate cancer.
- Phase 2 trial, NCT number: NCT03248570 2
These clinical trials and several others will allow further understanding of how immunotherapy treatment combined with other treatments can improve overall survival and quality of life in patients diagnosed with metastatic prostate cancer.
It is also important to consider that several patients are unable to participate in clinical trials. If a recruiting clinical trial is found, several barriers exist that prevent a patient from participating in a desired clinical trial. This included insurance issues, inability to travel to clinical trial locations, or difficulty submitting necessary information for consideration. Knowledge of barriers causing access to care and socioeconomic disparities is an essential component in the management of patients diagnosed with prostate cancer. Further investigations of these barriers will educate healthcare professional on what additional measures that should be implemented to provide quality patient care.
References:
- Immunotherapy for Prostate Cancer. Cancer Research Institute. https://www.cancerresearch.org/immunotherapy/cancer-types/prostate-cancer. Published 2020. Accessed August 17, 2020.
- Prostate Cancer – List Results. Home – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=Prostate+Cancer. Published 2020. Accessed August 17, 2020.